2024 Presidential Interdisciplinary Research Seed Grants Awarded
Funding to Support Investigations Into Functional Genomics, Metabolic Modeling
Caitlin Ware, Iowa State University Office of the Vice President for Research
Posted Feb 26, 2024
The Iowa State University Office of the Vice President for Research (OVPR) has awarded $100,000 in 2024 Presidential Interdisciplinary Research Seed Grant program (PIRS) funding to support university scholars exploring novel approaches to genomics studies and metabolic dysfunction.
Established in 2017, the PIRS program is administered by the OVPR and supported with funds from the Office of the President and an endowment from the Mary G. Miller estate. The annual award is given to support university faculty members pursuing initiatives that are innovative, high-risk, and high reward, with an interdisciplinary focus and strong potential for external funding.
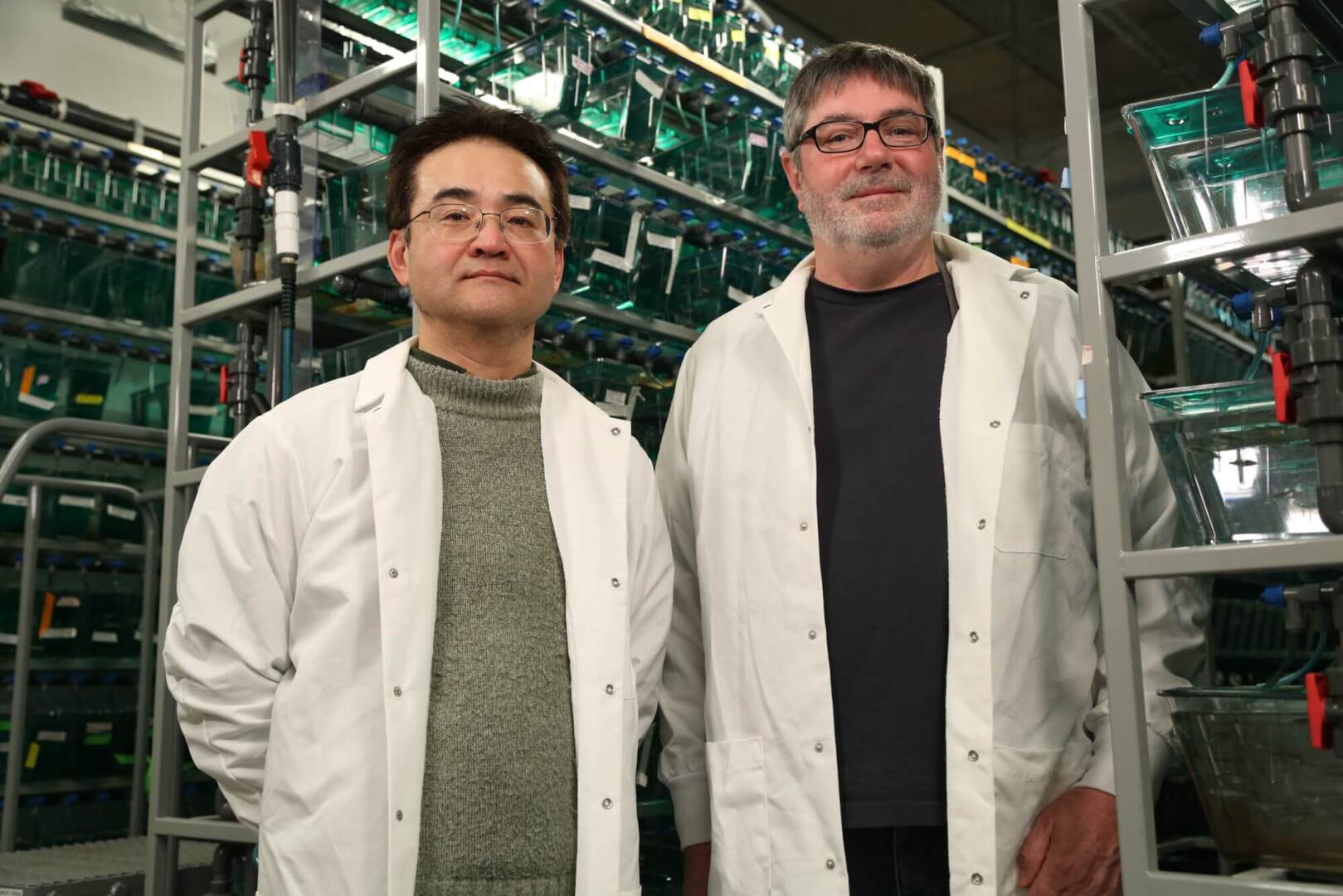
Two teams were recently selected to receive $50,000 each in 2024 PIRS funding, to be used over the course of two years. Professor of Chemistry Young-Jin Lee and professor of Genetics, Development, and Cell Biology Jeffrey Essner will use awarded institutional funding to investigate the use of mass spectrometry imaging as an innovative tool to study the metabolic activity of zebrafish embryos. With their PIRS funding, professor of Feedlot Nutrition Stephanie Hansen and associate professor of Veterinary Microbiology and Preventative Medicine and John G. Salsbury Endowed Chair in Veterinary Medicine Jodi McGill will collaborate to study the use of feedlot cattle as a model for human metabolic dysfunction and obesity.
“We’re delighted to provide PIRS funding to support the creative and innovative research of doctors Essner, Lee, McGill, and Hansen,” said Vice President for Research Peter Dorhout. “Their work truly embodies the purpose of PIRS funding – to support high-risk, high-reward projects that could turn into larger initiatives that create opportunities and forge new frontiers.”
A New Approach to Microscopic Imaging
Within the realm of functional genomics, the ultimate goal of scholarly work is to understand all gene functions within every genomic component of the organism being examined. To date, a variety of technologies have been developed to meet this broad goal, the majority of which focus on exploring DNA and RNA levels. However, a large knowledge gap currently exists in understanding gene expression and functions associated with specific organs and cells, such as why certain gene functions are only triggered under specific conditions. Through their PIRS project, titled, “Platform Technology for Functional Genomics: Spatially Resolved Isotope Tracing of Zebrafish,” Lee and Essner will combine their respective expertise in imaging technology and zebrafish genetics to explore the development of mass spectrometry imaging (MSI), designed to capture the organ-specific metabolic activity of zebrafish embryos.
Zebrafish are a preferred animal model system for a variety of genomics-focused studies, due to the inherent ease with which their genetics can be manipulated and the transparent nature of their embryos. They are also an ideal model for investigating human health conditions, as they share a similar organ structure. Together, Lee and Essner will create technology designed to directly monitor metabolic activity at the cellular and organ level in zebrafish. By combining isotope tracing — a technique that tracks certain atoms through a series of biochemical reactions — with a process known as high-spatial resolution mass spectrometry imaging, the duo plan to measure the metabolic activity linked to each tiny organ of zebrafish. The use of the proposed imagining technology is expected to allow cellular resolution of metabolic activity during development and disease progression, providing new inroads into understanding these processes in humans.
Utilizing the proposed MSI techniques will alleviate the need to individually study each zebrafish organ, which are oftentimes too small to handle successfully. Instead, molecules from each organ will be sampled, mass-measured, and isotope-traced at a microscopic level.
“I have always been fascinated by zebrafish. It is such a tiny fish, but it has such a wide range of utilities,” said Lee. “I am also fascinated by isotope tracing, and I thought that combining these interests could solve the problem associated with zebrafish being too tiny to study metabolism change in each organ. But I’m a technology guy. I don’t know much about biology, and certainly not zebrafish biology. The collaborative nature of this project and the partnership it involves means that technology and biology can be combined in a very exciting way.”
The project will focus on two key goals: studying the microscopic, organ-specific fructose metabolism of zebrafish embryos as a proof-of-concept study and developing customized data analysis protocols. Fructose was selected as the area of emphasis due to its implications for human health, as high fructose consumption is linked to various metabolic diseases, including non-alcoholic fatty liver disease (NAFLD), type 2 diabetes, and kidney dysfunction. The second research thrust of the project, data collection and analysis, will potentially establish a foundation for the future study of metabolic diseases or cancer metabolism influenced by genetics, environment, and nutrients.
“Any time you develop a new technology that allows you to look at something differently, and in this case to visualize something nobody else can, it opens up whole new areas of science,” Essner said. “As far as we are aware, nobody has studied the metabolism of zebrafish embryos in this detail before due to their small size. If we are successful, this preliminary work should have a very direct impact on human health. It will not only prove that our approach can decipher metabolism in the extremely small organs of zebrafish embryos, but also that zebrafish can serve as a good model for the study of metabolic disease in humans.”
A Feedlot Model for Metabolic Dysfunction

Obesity represents a rapidly expanding human health crisis globally, specifically due to the relationship between obesity and metabolic dysfunctions – with insulin resistance as a common resulting disorder. Within the U.S., the rising prevalence of diet-related diseases, such as type 2 diabetes, obesity, and hypertension, has been cited as a “health crisis” by the Biden-Harris Administration. But despite the growing awareness and concern linked to metabolic diseases, when it comes to addressing the science behind the upward trend in prevalence, current animal models for obesity and related pathologies have significant limitations. Most notably, in terms of being able to translate animal data into findings helpful in the treatment of human conditions.
Together, McGill and Hansen hope to address these limitations by laying the groundwork for using Iowa’s feedlot cattle industry as a new model for human metabolic dysfunction and obesity, in their PIRS project entitled, “Exploring Potential of Obese Feedlot Cattle as a Model for Diabetes in Humans.”
Built on similar physiological responses to high-calorie diets and low exercise shared by both humans and feedlot cattle, McGill and Hansen plan to investigate the relationship between body fat, inflammation, and metabolic changes as cattle progress from the growing to finishing stages of beef production. Because a growing body of evidence also suggests that feedlot cattle can experience declining insulin sensitivity – just as humans do when developing diabetes – the project will also include assessments of insulin sensitivity in cattle with a variety of obesity levels.
By exploring the interplay between body fat, inflammatory responses, and metabolic syndrome changes in a naturally occurring obesity model via feedlot cattle, McGill and Hansen’s PIRS initiative will attempt to fill a critical gap in traditional models, which often fail to accurately reflect human metabolic processes due to their differing lifespans, metabolisms, and sizes. Findings related to these dynamics hold the potential to inform future therapeutic interventions for a variety of human metabolic diseases.
“My passion is the immune system and how it works, and most of my expertise is in infectious diseases, making this project a pivot for looking at the immune system in a completely new context,” McGill said. “But I love a new challenge and this project is building the foundation for future collaborative work in this area. By leveraging our combined experience in large-animal models and immunometabolic processes, we hope to unlock a new avenue that has remained largely unexplored.”
In addition to the potential human health impacts of the research project, attention will be paid to the impacts of obesity on the beef production industry, in which there is a delicate balance between consumer-preferred marbling scores and incentive for an animal to reach a higher body weight for greater economic return. By evaluating the progression of metabolic dysfunction as cattle increase in weight and body fat percentages, data will be collected related to cattle metabolism and efficiency of nutrient use.
“The translational potential of this research is significant, as it can inform both practices for managing cattle health and growth and offer groundbreaking perspectives in human medical interventions,” Hansen said. “I am really excited to study these late-stage finishing cattle and determine if we can continue to improve efficiency of feed conversion to beef, which would mean fewer resources needed for every pound of beef we produce and greater profitability for stakeholders in the state.”
The Presidential Interdisciplinary Research Seed Grant Program (PIRS) is open to full-time, tenured/tenure-eligible, and term faculty with the rank of assistant professor, assistant teaching professor, clinical assistant professor, and adjunct assistant professor, or higher ranking faculty from any discipline. PIRS supports the initial development of innovative research, with up to $50,000 in funding designated for selected teams to pursue high-risk, high-reward projects over a two-year period. The awards are designed to support projects that help researchers from different disciplines collaborate on groundbreaking research, including collecting data, organizing workshops, and building partnerships with other organizations.